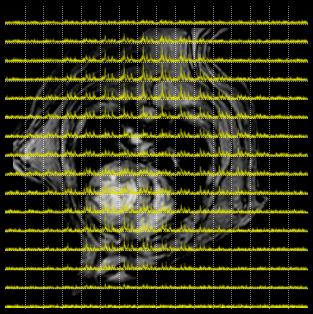
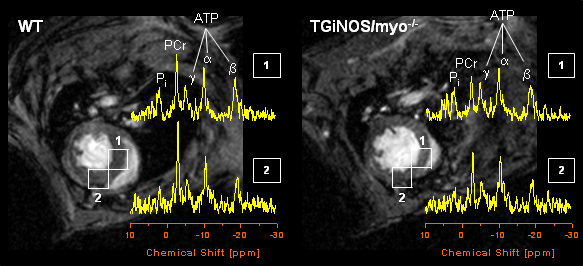
Determination of energy carriers by 31P NMR spectroscopy
|
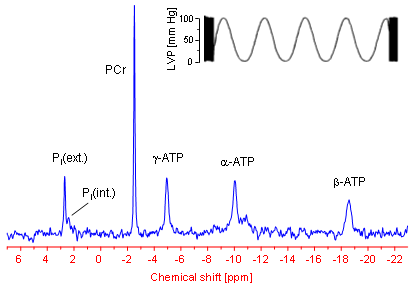
|
|
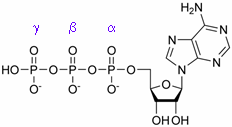
In order to ensure a continuous beating of the heart, its energy supply has to be reliably regulated. The most important energy carrier within the body is ATP; its chemical structure is shown in the margin. This molecule can be excellently detected by 31P NMR spectroscopy – each of its three phosphorus atoms yields a unique signal, as can be seen in the figure on the right hand side.
|
|
Adenosine triphosphate (ATP)
|
|
Within a 31P NMR spectrum of an isolated perfused mouse heart additional signals for phosphocreatine (PCr) as well as for intra- und extracellular inorganic phosphate (labelled with Pi(int.) and Pi(ext.), respectively) can be identified. In particular the position of the latter signals in the spectrum (the chemical shift) strongly depends from the pH value of the tissue environment, which can be used to easily calculate (→ more details) both the extracellular and cytosolic pH (pHe and pHi, respectively).
|
|
For a long time it was accepted that solely the absolute amount of ATP is the crucial driving force for the energy-consuming cellular processes. However, in the meantime there is general agreement that not only the ATP concentration, but specifically the ratio of ATP to its breakdown products ADP and Pi is the decisive determinant for the amount of energy released by ATP hydrolysis (ATP ↔ ADP + Pi). The intracellular ADP concentration can be calculated from the 31P NMR spectrum of the heart via the creatine kinase equilibrium (PCr + ADP ↔ ATP + Cr). Under normal conditions the amount of ADP is about two orders of magnitude lower than the cytosolic ATP level (~30 µM ADP compared with ~6 mM ATP). Thus, by means of 31P NMR spectroscopy all parameters required to assess the amount of energy available to the heart (ΔGATP) can be continuously and non-invasively monitored (→ more details).
In parallel to the energetics, cardiac pump function can be determined via a balloon introduced into the left ventricle and attached to a pressure transducer. A characteristic pressure registration obtained with this setup is illustrated in the upper part of the 31P NMR spectrum. The zoomed area shows clearly the periodical pressure alterations during the heart action. The minima of the trace reflect the end-diastolic and the maxima the end-systolic pressure, respectively – the difference [maximum - minimum] yields the developed pressure of the heart. The distance between the minima or maxima can be used to calculate the heart rate, which is found to be tenfold higher in the mouse (~600 beats per minute) than in man.
|
|
|
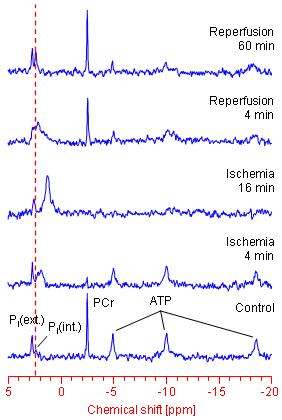
31P NMR spectroscopy is especially suitable to study alterations of the myocardial energy metabolism when the supply of the heart is interrupted. This may occur by a sudden occlusion of one of the coronary arteries such as during a heart attack. This for man extremely dangerous event can be simulated in the laboratory with the isolated heart preparation by stopping the buffer supply. The period without perfusion is referred to as ischemia, the period after restoration of the supply as reperfusion.
|
|
|
|
As can be clearly seen in the left figure, immediately after interruption of the heart supply a drop in the PCr and a rise in the Pi(int.) level are observed. With reference to the dotted red line a high field shift of the Pi(int.) signal can be concomitantly made out, which reflects the acidification of the muscle tissue due to the limited perfusion. On the other hand, an absolute ATP depletion occurs only after 15 minutes – even at a complete stop of the buffer supply.
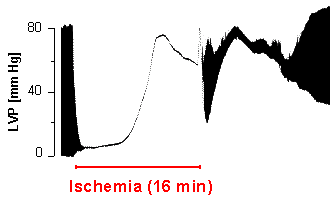
In parallel to the exhaustion of cardiac energy stores left ventricular pressure development dwindles and thereby also the pump function of the heart (figure at the right). Very quickly contractile function completely grinds to a halt, and a bit later the so-called contracture develops (pressure increase during ischemia), which is attributed to the inundation of the cytosol by Ca2+ ions. The latter is caused by breakdown of the membranous ion gradients and failure of ATP-driven ion pumps, respectvely, as a consequence of the impaired energy state.
|
|
When the supply of the heart is re-established, the PCr level already recovers after a couple of minutes. Immediately after restoration of the perfusion an "overshoot" of PCr over the basal value (known as PCr overshoot) is frequently observed. In contrast, ATP levels often do not reach their initial values due to loss of purines during ischemia and the early reperfusion period, respectively. After normalization of cardiac pHi also the contractile parameters get more and more stabilized.
To what extent the original heart function can be recovered depends from the duration of ischemia and the applied "therapy" in terms of additionally infused drugs or the transgenic model used. For example, we could show that hearts without endothelial nitric monoxid synthase (eNOS knockout mice) recover better from an ischemic period than the corresponding control hearts, whereas on the other hand the lack of myoglobin has an adverse effect (compare also the original publications given below). Thus, by an appropriate combination of transgenic animal model and pharmacologic intervention experimental series can be carried out, that may give a clue for a more effient therapy of heart attack in man.
|
Top |
In vivo 2D mapping of the energy state
|
|
Top |
Own work about cardiac energetics and function
|
|
| A complete overview about our peer-reviewed publications of the last years can be found here. The references are linked with the PubMed abstracts of the National Library of Medicine. If your are interested in one of these papers, and you don't have online access to the respective journal, send us an email, so that we can provide you with the appropriate pdf-file. |
| |
| Methodical studies |
| |
| Flögel U, Jacoby C, Gödecke A, Schrader J. |
| In vivo 2D mapping of impaired murine cardiac energetics in NO-induced heart failure. |
| Magn Reson Med. 2007; 57: 50-8. |
| |
| Flögel U, Decking UK, Gödecke A, Schrader J. |
| Contribution of NO to ischemia-reperfusion injury in the saline-perfused heart: a study in endothelial NO synthase knockout mice. |
| J Mol Cell Cardiol. 1999; 31: 827-36. |
| |
| Applications |
| |
| Haddad S, Wang Y, Galy B, Korf-Klingebiel M, Hirsch V, Baru AM, Rostami F, Reboll MR, Heineke J, Flögel U, Groos S, Renner A, Toischer K, Zimmermann F, Engeli S, Jordan J, Bauersachs J, Hentze MW, Wollert KC, Kempf T. |
| Iron-regulatory proteins secure iron availability in cardiomyocytes to prevent heart failure. |
| Eur Heart J. 2017; 38: 362-372. |
|
| Tucci S, Flögel U, Hermann S, Sturm M, Schäfers M, Spiekerkoetter U. |
| Development and pathomechanisms of cardiomyopathy in very long-chain acyl-CoA dehydrogenase deficient VLCAD(-/-) mice. |
| Biochim Biophys Acta 2014; 1842: 677-85. |
| |
| Luedde M, Flögel U, Knorr M, Grundt C, Hippe HJ, Brors B, Frank D, Haselmann U, Antony C, Voelkers M, Schrader J, Most P, Lemmer B, Katus HA, Frey N. |
| Decreased contractility due to energy deprivation in a transgenic rat model of hypertrophic cardiomyopathy. |
| J Mol Med. 2009; 87: 411-22. |
| |
| Rassaf T, Flögel U, Drexhage C, Hendgen-Cotta U, Kelm M, Schrader J. |
| Nitrite reductase function of deoxymyoglobin: oxygen sensor and regulator of cardiac energetics and function. |
| Circ Res. 2007; 100: 1749-54. |
| |
| Flögel U, Gödecke A, Klotz LO, Schrader J. |
| Role of myoglobin in the antioxidant defense of the heart. |
| FASEB J. 2004; 18: 1156-8. |
| |
| Warskulat U, Flögel U, Jacoby C, Hartwig HG, Thewissen M, Merx MW, Molojavyi A, Heller-Stilb B, Schrader J, Häussinger D. |
| Taurine transporter knockout depletes muscle taurine levels and results in severe skeletal muscle impairment but leaves cardiac function uncompromised. |
| FASEB J. 2004; 18: 577-9. |
| |
| Wunderlich C, Flögel U, Gödecke A, Heger J, Schrader J. |
| Acute inhibition of myoglobin impairs contractility and energy state of iNOS-overexpressing hearts. |
| Circ Res. 2003; 92: 1352-8. |
| |
| Gödecke A, Molojavyi A, Heger J, Flögel U, Ding Z, Jacoby C, Schrader J. |
| Myoglobin protects the heart from inducible nitric-oxide synthase iNOS-mediated nitrosative stress. |
| J Biol Chem. 2003; 278: 21761-6. |
| |
| Merx MW, Flögel U, Stumpe T, Gödecke A, Decking UK, Schrader J. |
| Myoglobin facilitates oxygen diffusion. |
| FASEB J. 2001;15: 1077-9. |
| |
| Flögel U, Merx MW, Gödecke A, Decking UK, Schrader J. |
| Myoglobin: A scavenger of bioactive NO. |
| Proc Natl Acad Sci USA. 2001; 98: 735-40. |
| |
| Gödecke A, Flögel U, Zanger K, Ding Z, Hirchenhain J, Decking UK, Schrader J. |
| Disruption of myoglobin in mice induces multiple compensatory mechanisms. |
| Proc Natl Acad Sci USA. 1999; 96: 10495-500. |
| |