
 |
| | ||
| About fluorine | Imaging via the fluorine nucleus | |
| Inflammation | ||
| Heart | ||
| Brain | ||
| Publications | ||
|
Why fluorine? |
||
|
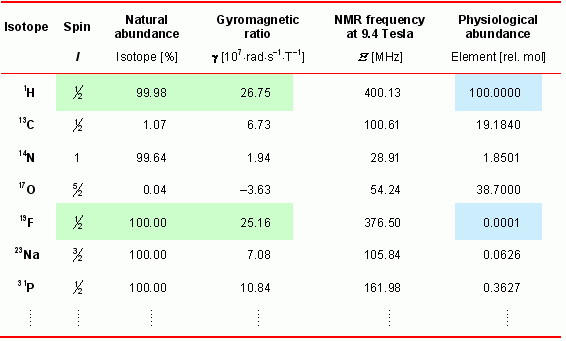
The table at the right lists some MR-relevant properties of isotopes from biological important elements. For MR sensitivity of a nucleus its gyromagnetic ratio is the most important parameter. As can be seen in the table, the naturally occurring stable fluorine isotope 19F (100%) is MR active and exhibits a sensitivity close to the 1H nucleus which is used routinely for anatomical and functional MR imaging. However, at the same time its abundance in the organism is negligible (with traces in bones and teeth).
Because of the lack of any 19F background in the body, detected signals from injected 19F-containing compounds are highly specific. An exact anatomical localization of fluorinated substances within the organism can easily obtained by acquisition of morphological matching 1H and 19F MR images and subsequent merging of the anatomical corresponding images (see below). |
|
|
| Top |
Discovering foci of inflammation with perfluorocarbons |
| Inflammation is associated with a large number of human diseases such as atherosclerosis, glomerulonephritis, inflammatory bowel disease, transplant rejection, neurodegenerative brain diseases, brain and spinal cord trauma, myocarditis, and ischemic heart disease. Thus, the medical problem is vast and an exact diagnosis is often difficult. Accordingly, therapy frequently is limited to symptomatic treatment and the success of the prescribed therapy is difficult to assess. Although recent advances involve various imaging modalities such as PET, CT, MRI, optical imaging, and ultrasound imaging, the visualization of inflammatory processes still poses a serious challenge, especially because in the initial phase the affected tissue does not exhibit specific physical properties that can be used to create contrast between inflamed and healthy regions. | ||
|
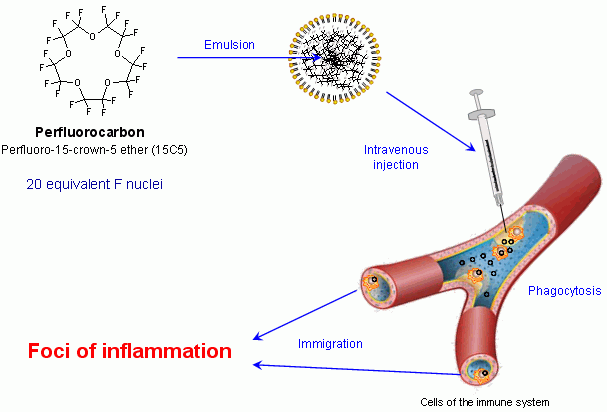
For this reason we developed a new technique (see scheme) to detect inflammatory processes with an unequivocal positive contrast via mittels 19F MRI. As contrast agent we used nanoparticles containing perfluorocarbons (PFCs), a family of compounds known to be biochemically inert (e.g. Teflon). Some of the PFC members such as perfluorodecalin, perfluorotripropylamine, perfluorodichloroctane, and perfluorooctyl bromide (also known as perflubron or Oxygent®) were already used in patients as artificial blood substitutes.
However, in our present investigations we used perfluoro-15-crown-5 ether, a PFC in which all 20 fluorine nuclei are chemically and magnetically equivalent (see chemical structure at the left) and thus exhibits superior properties for 19F MRI detection. This substance is a liquid which is completely insoluble in water and, therefore, must be emulsified for application into the circulation. The particle size of the emulsion was chosen to ensure an optimal uptake by cells of the organism's immune defense. |
|
| After intravenous administration of the emulsified PFCs indeed an efficient and selective uptake of the contrast agent by circulating cells of the monocyte-macrophage-system (see scheme above) takes place as confirmed by comprehensive analysis of tissue and blood samples (→ more details) after PFC injection (Flögel et al, 2008). The infiltration of the 19F-loaded, immunocompetent cells into inflamed tissue areas then permits the unambiguous identification of affected regions in vivo by combined 1H/19F MRI. This is illustrated below at two independent acute inflammation models in the mouse. | ||
| Top |
Monitoring of acute inflammation after myocardial infarction |
|
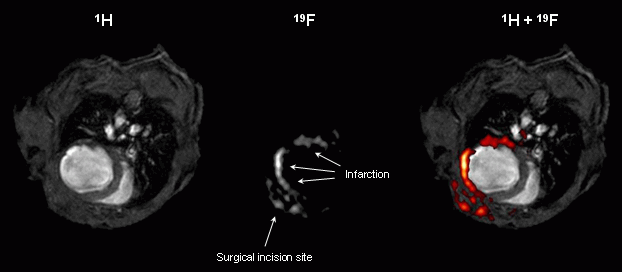
The figures at the right show MR images from the thorax of a mouse after induction of myocardial infarction by occlusion of a coronary artery – a procedure well known to be associated with an acute inflammatory response in the affected tissue.
The upper row illustrates the principle of the combined 1H/19F MRI approach: The end-diastolic 1H image (left) clearly shows the presence of ventricular dilatation and wall thinning within the infarcted area, and in the corresponding 19F image (middle), a signal pattern matched the shape of the free left ventricular wall. Merging of these images (right) confirms the localization of PFCs within the anterior, lateral, and posterior walls. Furthermore, 19F signal also was detected in the adjacent chest tissue, where thoracotomy for the surgical intervention was performed. Note that otherwise no background 19F signal from other tissue is present. MRI data were confirmed by histology using PFCs additionally labelled with rhodamine. Immunohistochemisty further revealed the fluorescence to be associated with macrophages in the infarcted tissue (Flögel et al, 2008). |
|
|
|
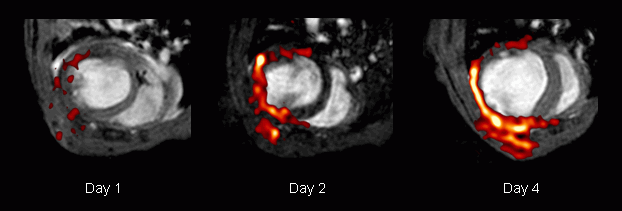
Repetitive measurements from day 1 after ligation of the coronary artery revealed a time-dependent accumulation of PFCs within the infarcted region as shown in a representative example in the lower row. The end-diastolic 1H images acquired 1, 2, and 4 days after induction of myocardial infarction show the progressive left ventricular dilatation as a consequence of the insult. Merging with the matching 19F images (red) demonstrates the successive infiltration of PFCs into the affected area of the heart and the region of the chest injured by surgery. Detected 19F signals were restricted to the area near the infarcted region of the heart; at no time were infiltrating PFCs observed within the septum.
Although strong PFC signals were found in ex vivo 19F images of blood components (→ see here), in vivo signals from PFCs in the circulation were not detectable at all (e.g. no signal within ventricular chambers; see figure above). Even when 19F images were acquired immediately after injection, no 19F signal from the streaming blood could be observed because the pulse sequence used for 19F MRI (RARE, Rapid Acquisition with Relaxation Enhancement) results in a signal void of flowing blood particles. Therefore, detected signals can be attributed unequivocally to accumulated PFCs in the tissue without contamination from 19F signals of circulating PFCs. |
||
| Nach oben |
Visualization of inflammation after cerebral ischemia |
|
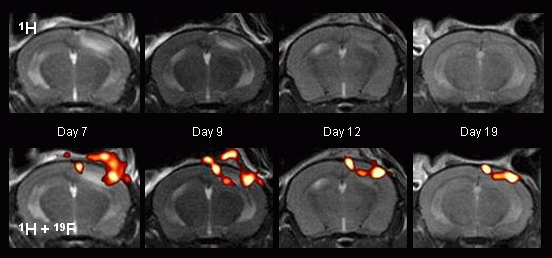
In another set of experiments, focal cerebral ischemia was chosen as an additional model of acute inflammation. After ischemia was induced by photothrombosis, mice were imaged at regular intervals up to 4 weeks after surgery. In RARE 1H images, the ischemic region appeared initially as a bright area (see adjacent figure, top left), and the corresponding 19F images clearly show infiltration of PFCs into the border zone of the infarct, which was detected at the earliest at day 4 after photothrombosis.
19F signal also was transiently observed supracranially at the location of skin incision (bottom left). Characteristic 1H and 19F images acquired from an individual mouse 7, 9, 12, and 19 days after focal cerebral ischemia was induced definitely show movement of the PFCs with the rim of the shrinking infarct over time (bottom). |
|
|
To support the notion that PFCs were carried into the ischemic region by monocytes/macrophages, experiments with rhodamine-labelled PFCs were again conducted. Microscopic survey images after immunostaining for monocyte/macrophage marker exhibited a pattern of fluorescence comparable to that observed for the 19F signal in the preceding MR experiment. Furthermore, comparison of fluorescence at large magnification indicated colocalization of PFCs and monocytes/macrophages (Flögel et al, 2008). Moreover, it is of note that in the two experimental models the data obtained by our combined 1H/19F MRI approach – for both the time course of accumulation and the localization of PFC-containing monocytes/macrophages within ischemic areas – are in good agreement with previous reports on myocardial and cerebral infarction.
In summary, the results show that intravenously applied PFCs accumulate after phagocytosis in inflammatory areas, where they can be detected by 1H/19F MRI. PFCs, therefore, can serve as a “positive” contrast agent for the detection of inflammation by MRI, permitting a spatial resolution close to the anatomic 1H image and an excellent degree of specificity resulting from the lack of any 19F background. Because PFCs are nontoxic, this approach may have a broad application in the imaging and diagnosis of numerous inflammatory disease states. |
||
| Nach oben |
Own work about fluorine imaging |
| A complete overview about our peer-reviewed publications of the last years can be found here. The references are linked with the PubMed abstracts of the National Library of Medicine. If your are interested in one of these papers, and you don't have online access to the respective journal, send us an email, so that we can provide you with the appropriate pdf-file. |
|
| Methodical studies |
| Temme S, Grapentin C, Quast C, Jacoby C, Grandoch M, Ding Z, Owenier C, Mayenfels F, Fischer JW, Schubert R, Schrader J, Flögel U. |
| Noninvasive imaging of early venous thrombosis by 19F magnetic resonance imaging with targeted perfluorocarbon nanoemulsions. |
| Circulation. 2015; 131: 1405-14. |
| Jacoby C, Temme S, Mayenfels F, Benoit N, Krafft MP, Schubert R, Schrader J, Flögel U. |
| Probing different perfluorocarbons for in vivo inflammation imaging by 19F MRI: image reconstruction, biological half-lives and sensitivity. |
| NMR Biomed. 2014; 27: 261-71. |
| Flögel U, Ding Z, Hardung H, Jander S, Reichmann G, Jacoby C, Schubert R, Schrader J. |
| In vivo monitoring of inflammation after cardiac and cerebral ischemia by fluorine magnetic resonance imaging. |
| Circulation. 2008; 118: 140-8. |
| Reviews and book chapters |
| Güden-Silber T, Temme S, Jacoby C, Flögel U. |
| Biomedical 19F MRI using perfluorocarbons. |
| Methods Mol Biol. 2018; 1718: 235-257. |
| |
| Temme S, Grapentin C, Güden-Silber T, Flögel U. |
| Active targeting of perfluorocarbon nanoemulsions. |
| In: Fluorine Magnetic Resonance Imaging. Edited by Flögel U, Ahrens E; Pan Stanford Publishing, Singapore. 2016: 103-39. |
| Grapentin C, Mayenfels F, Barnert S, Süss, Schubert R, Temme S, Jacoby C, Schrader J, Flögel U. |
| Optimization of perfluorocarbon nanoemulsions for molecular imaging by 19F MRI. |
| In: Nanomedicine. Edited by Seifalin A, de Mel A, Kalaskar DM; One Central Press, Manchester. 2014: 268-86. |
| Temme S, Bönner F, Schrader J, Flögel U. |
| 19F magnetic resonance imaging of endogenous macrophages in inflammation. |
| Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2012; 4: 329-43. |
| Applications |
| Flögel U, Schlüter A, Jacoby C, Temme S, Banga JP, Eckstein A, Schrader J, Berchner-Pfannschmidt U. |
| Multimodal assessment of orbital immune cell infiltration and tissue remodeling during development of graves disease by 1H/19F MRI. |
| Magn Reson Med. 2018; 80: 711-718. |
| |
| Ding Z, Temme S, Quast C, Friebe D, Jacoby C, Zanger K, Bidmon HJ, Grapentin C, Schubert R, Flögel U, Schrader J. |
| Epicardium-derived cells formed after myocardial injury display phagocytic activity permitting in vivo labeling and tracking. |
| Stem Cells Transl Med. 2016; 5: 639-650. |
| |
| Temme S, Jacoby C, Ding Z, Bönner F, Borg N, Schrader J, Flögel U. |
| Monitoring the trafficking of neutrophil granulocytes and monocytes during the course of tissue inflammation by noninvasive 19F MRI. |
| J Leukoc Biol. 2014; 95: 689-97. |
| Jacoby C, Borg N, Heusch P, Sauter M, Bönner F, Kandolf R, Klingel K, Schrader J, Flögel U. |
| Visualization of immune cell infiltration in experimental viral myocarditis by 19F MRI in vivo. |
| Magn Reson Mater Phy. 2014; 27: 101-6. |
| Flögel U, Burghoff S, van Lent PL, Temme S, Galbarz L, Ding Z, El-Tayeb A, Huels S, Bönner F, Borg N, Jacoby C, Müller CE, van den Berg WB, Schrader J. |
| Selective activation of adenosine A2A receptors on immune cells by a CD73-dependent prodrug suppresses joint inflammation in experimental rheumatoid arthritis. |
| Sci Transl Med. 2012; 4: 146ra108. |
| Flögel U, Su S, Kreideweiß I, Ding Z, Galbarz L, Fu J, Jacoby C, Witzke O, Schrader J. |
| Noninvasive detection of graft rejection by in vivo 19F MRI in the early stage. |
| Am J Transplant. 2011; 11: 235-44. |
| |
| Ebner B, Behm P, Jacoby C, Burghoff S, French BA, Schrader J, Flögel U. |
| Early assessment of pulmonary inflammation by 19F MRI in vivo. |
| Circ Cardiovasc Imaging. 2010; 3: 202-10. |
|
|||