
 |
| | ||
| Methodology | Cardiac Imaging in the Mouse | |
| Data analysis | ||
| Infarction | ||
| Publications | ||
|
Experimental details |
||
|
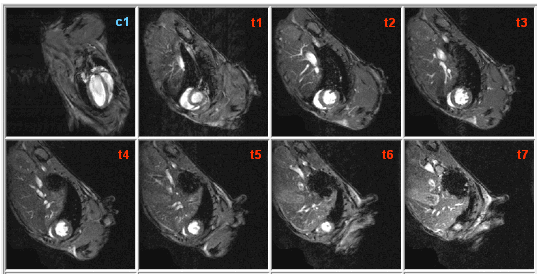
Axial slice |
To obtain information on the morphology and functional parameters of transgenic mice heart, we established standard protocols that enable us to rapidly acquire high quality images. Currently, it is possible to carry out all measurements necessary for the determination of end-diastolic, end-systolic, and stroke volumes, ejection fraction, cardiac output, left-ventricular mass and wall thickness within less than 60 minutes.
Anesthesia: Mice are anesthetized within the magnet using 1.5% isofluran in air (see Hardware) applied at a rate of 75 ml/min using a home-build nose cone. Injection narcosis (e.g. Hypnorm/Diazepam) is disadvantageous due to insufficient controllability as well as respiratory and cardio-depressive side effects of these substances. In contrast, using inhalation anesthesia as per description, physiological heart and respiration rates of ~600 min-1 and ~100 min-1, respectively, can be maintained for more than 1 hour. This is important to obtain reliable information on murine heart function in vivo. Temperature control: Furthermore, it is important to maintain the body temperature of the mouse (37 °C) during the whole measurement. Of course, no heating coils should be brought into the magnet, so that an alternative way is needed to control temperature. Therefore, we set the magnet gradient cooling system (coupled to a water recirculator Haake UWK 45) to body temperature which is unproblematic, unless the pulse sequence used does not extremely heat up the gradient system. For these cases, we developed a water cooling system with a special profile flexible tube that ensures a very high thermal transfer. The thereby produced water signal can be eliminated by a saturation module integrated into the pulse sequence. A thermocouple (Pt10Rh-Pt) is used to rectally monitor the animal's body temperature. ECG and respiration gating: Gating is realized by a trigger module (SA Instruments M 1025) directly connected to the spectrometer. To obtain images at defined time points within the heart cycle, triggering of the image sequence (FLASH, Fast Low Angle Shot) to an ECG signal is necessary. This signal can be derived by fixing fore- and hindpaws to small pediatric electrodes. Furthermore, a pressure sensor is used to avoid respiratory motion artifacts by synchronization of the expiratory signal plateau with the pulse sequence. Only QRS complexes (triggering to end-diastolic phase) within this plateau are used to initialize a data acquisition cycle. During a complete heart cycle we usually acquire 15 to 20 images resulting in a temporal resolution of 5-7 ms (assuming a heart rate of 600 min-1). A field of view of 3x3 cm2 and a matrix size of 256x256 yield a pixel size of 117x117 µm (at a slice thickness of 1 mm). Temporal and spatial resolution are completely sufficient to routinely determine functional heart parameters (see movies on the left). Orthostasis: To exclude that the vertical position of the mouse during the measurement affects the physiological parameters, we monitored hemodynamic and contractile parameters of isofluran-anestethized mice for more than 1 hour (corresponding to the total acquisition time for heart imaging). These data provided by a 1.4 F Millar catheter showed that the vertical position of the mouse has no significant effect on the results. |
|
|
Coronal Slice |
||
|
While analysis of the MR images (see next section) provides important parameters for the assessment of heart function, we are additionally able to get in vivo information about heart energetics without changing the experimental setup. Spatial resolved 31P-NMR spectroscopy is used to assess the energetic situation in the mouse heart by means of phosphocreatine and ATP signal intensities. A more detailed description of this method can be found under "Energetics" in the section In vivo 2D mapping of the energy state. |
| Top |
MR data analysis |
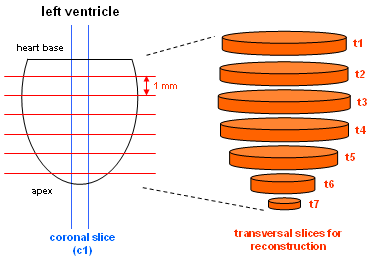
| Usually a single coronal as well as 6 to 8 transverse tomographic images are used to determine left ventricle (LV) volumes. While the coronal slice (c1, see figure below) is only important for correction of the partial volume of the apex slice (for more information see here), LV volume reconstruction is performed using the transverse slices t1-t7. As can be seen from the figure, the left ventricle is dissected into contiguous tomographic slices of 1 mm thickness whose partial volumes are determined and summed afterwards. |
|
|
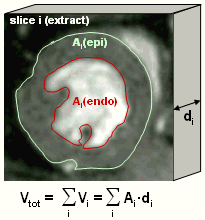
| For each slice 15-20 images (so-called "frames") covering a complete heart cycle are obtained, and for each of the respective points of time we can calculate the LV volume. For determination of functional heart parameters, we are solely interested in end-diastolic and end-systolic volumes. By triggering to the QRS complex of the ECG the end-diastolic image is automatically given by the first frame, whereas end-systole has to be found by inspection. It corresponds to maximal contraction and therefore to minimal endocardial area. |
|
For all slices end-diastolic and end-systolic left ventricular endocardial areas are planimetrically determined (red line in the left figure) which, after multiplication with the slice thickness, provide the partial volumes. End-diastolic (EDV) and end-systolic (ESV) volumes are obtained by summation of all partial volumes associated with the respective heart phases. The difference between EDV and ESV is identical to the stroke volume (SV) which is the amount of blood pumped into the periphery during a single heart cycle. Since SV depends on the total LV size, it is not appropriate to characterize the cardiac pump function. A more reliable parameter is the ejection fraction (EF) which is defined as SV/EDV (in %). The amount of blood delivered per time unit is called cardiac output (CO) and defined by the product of SV and heart rate (HR) which is recorded during the measurement. From the epicardial border (green line) the complete LV volume can be reconstructed in the same way described for the endocardial volume. Subtracting these two volumes provides the pure myocardial volume and, after multiplication with density, the myocardial mass. |
|
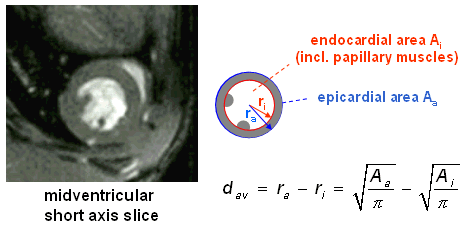
The myocardial wall thickness can be directly measured but choosing the positions for this measurement seems to be quite arbitrary. Therefore, averaging several so determined values may not provide reliable results. In addition to an average value of 3 length measurements within the septum, we use another method for the determination of a wall thickness averaged over the complete midventricular slice.
As can be seen from the figure on the right, both endo- and epicardial areas are approximated by circles, and the difference between the two radii provides an averaged wall thickness for the midventricular slice. Here, the papillary muscles have to be included into the endocardial area, whereas they have to be excluded for LV volume determination, since they are assigned to the myocardium and therefore not to the endocardial area. |
|
|
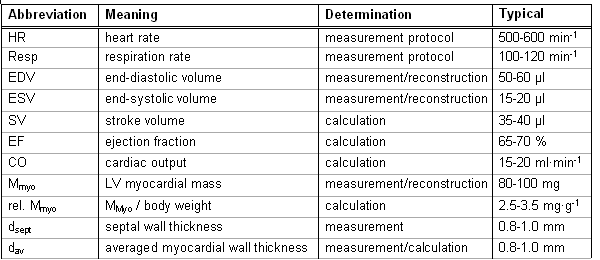
In the table we summarized the most important functional parameters, which can be derived from the analysis of MR images of the mouse heart.
Typical values for mouse hearts are given in the last column. It has to be mentioned that these were obtained from C57BL/6 wild type mice and that they can considerably vary for different strains. |
| Top |
Myocardial infarct |
|
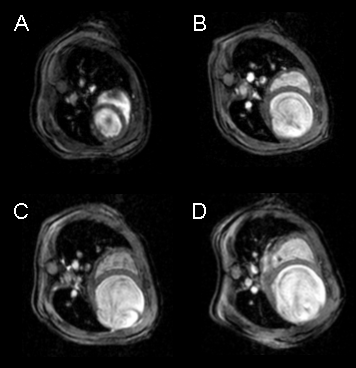
One of the main advantages of MR imaging is its non-invasivity which is especially important for repetitive measurements on the same animal, e.g. to follow a course of disease. An impressive example is presented by an experimental myocardial infarct.
In cooperation with the group of Prof. Dr. H. Drexler (MHH Hannover, Cardiology and Angiology) we consecutively investigated mice with mycardial infarction which was induced by occlusion of the LAD (left anterior descending coronary artery) under open-chest conditions and isoflurane inhalation anesthesia. Transverse slice images of a mouse heart before (A) and 1 (B) , 2 (C), and 4 (D) weeks after ligation of the LAD show that the progression of the myocardial infarct can easily be monitored by successive MR measurements. Furthermore, changes in the dynamics of LV contraction can directly be assessed using the cine sequence that is acquired for each heart slice. As can be seen the initially normal heart dilates from week to week after induction of the myocardial infarct. Furthermore, the ventricular wall obviously becomes very thin in the region of the ligated LAD (right bottom). Watching the movie of the cine sequence, it is clearly seen that during systole, almost no contraction of the myocardium can be observed. This leads to a strongly reduced ejection fraction of less than 20 %. Surprisingly, despite these limitations in heart function, the animals are moving (climbing!) within their cages without any obvious handicap. |
| Top |
Own work about cardiac imaging |
| A complete overview about our peer-reviewed publications of the last years can be found here. The references are linked with the PubMed abstracts of the National Library of Medicine. If your are interested in one of these papers, and you don't have online access to the respective journal, send us an email, so that we can provide you with the appropriate pdf-file. | |
| |
| Methodical studies | |
| Haberkorn SM, Jacoby C, Ding Z, Keul P, Bönner F, Polzin A, Levkau B, Schrader J, Kelm M, Flögel U. | |
| Cardiovascular magnetic resonance relaxometry predicts regional functional outcome after experimental myocardial infarction. | |
| Circ Cardiovasc Imaging. 2017; 10: pii e006025 (12 pages). | |
| | |
| Bönner F, Jacoby C, Temme S, Borg N, Ding Z, Schrader J, Flögel U. | |
| Multifunctional MR monitoring of the healing process after myocardial infarction. | |
| Basic Res Cardiol. 2014; 109: 430. | |
| Borst O, Ochmann C, Schönberger T, Jacoby C, Stellos K, Seizer P, Flögel U, Lang F, Gawaz M. | |
| Methods employed for induction and analysis of experimental myocardial infarction in mice. | |
| Cell Physiol Biochem. 2011; 28: 1-12. | |
| Flögel U, Ding Z, Hardung H, Jander S, Reichmann G, Jacoby C, Schubert R, Schrader J. | |
| In vivo monitoring of inflammation after cardiac and cerebral ischemia by fluorine magnetic resonance imaging. | |
| Circulation. 2008; 118: 140-48. | |
| Flögel U, Jacoby C, Gödecke A, Schrader J. | |
| In vivo 2D mapping of impaired murine cardiac energetics in NO-induced heart failure. | |
| Magn Reson Med. 2007; 57: 50-8. | |
| |
| Jacoby C, Molojavyi A, Flögel U, Merx MW, Ding Z, Schrader J. | |
| Direct comparison of magnetic resonance imaging and conductance microcatheter in the evaluation of left ventricular function in mice. | |
| Basic Res Cardiol. 2006; 101: 87-95. | |
| Applications | |
| Heinen A, Raupach A, Behmenburg F, Hölscher N, Flögel U, Kelm M, Kaisers W, Nederlof R, Huhn R, Gödecke A. | |
| Echocardiographic analysis of cardiac function after infarction in mice: Validation of single-plane long-axis view measurements and the bi-plane Simpson method. | |
| Ultrasound Med Biol. 2018; 44: 1544-1555. | |
| | |
| Erkens R, Suvorava T, Sutton TR, Fernandez BO, Mikus-Lelinska M, Barbarino F, Flögel U, Kelm M, Feelisch M, Cortese-Krott MM. | |
| Nrf2 deficiency unmasks the significance of nitric oxide synthase activity for cardioprotection. | |
| Oxid Med Cell Longev. 2018; 2018:8309698 (15 pages). | |
| | |
| Borg N, Alter C, Görldt N, Jacoby C, Ding Z, Steckel B, Quast C, Bönner F, Friebe D, Temme S, Flögel U, Schrader J. | |
| CD73 on T-cells orchestrates cardiac wound healing after myocardial infarction by purinergic metabolic reprogramming. | |
| Circulation. 2017; 136: 297-313. | |
| | |
| Behmenburg F, Hölscher N, Flögel U, Hollmann MW, Heinen A, Huhn R. | |
| Opening of calcium-activated potassium channels improves long-term left-ventricular function after coronary artery occlusion in mice. | |
| Int J Cardiol. 2017; 241: 351-357. | |
| | |
| Hendgen-Cotta UB, Esfeld S, Coman C, Ahrends R, Klein-Hitpass L, Flögel U, Rassaf T, Totzeck M. | |
| A novel physiological role for cardiac myoglobin in lipid metabolism. | |
| Sci Rep. 2017; 7: 43219 (13 pages). | |
| | |
| Haddad S, Wang Y, Galy B, Korf-Klingebiel M, Hirsch V, Baru AM, Rostami F, Reboll MR, Heineke J, Flögel U, Groos S, Renner A, Toischer K, Zimmermann F, Engeli S, Jordan J, Bauersachs J, Hentze MW, Wollert KC, Kempf T. | |
| Iron-regulatory proteins secure iron availability in cardiomyocytes to prevent heart failure. | |
| Eur Heart J. 2017; 38: 362-372. | |
| | |
| Rammos C, Hendgen-Cotta UB, Totzeck M, Pohl J, Lüdike P, Flögel U, Deenen R, Köhrer K, French BA, Gödecke A, Kelm M, Rassaf T. | |
| Impact of dietary nitrate on age-related diastolic dysfunction. | |
| Eur J Heart Fail. 2016; 18: 599-610. | |
| | |
| Ding Z, Temme S, Quast C, Friebe D, Jacoby C, Zanger K, Bidmon HJ, Grapentin C, Schubert R, Flögel U, Schrader J. | |
| Epicardium-derived cells formed after myocardial injury display phagocytic activity permitting in vivo labeling and tracking. | |
| Stem Cells Transl Med. 2016; 5: 639-650. | |
| | |
| Tucci S, Flögel U, Hermann S, Sturm M, Schäfers M, Spiekerkoetter U. | |
| Development and pathomechanisms of cardiomyopathy in very long-chain acyl-CoA dehydrogenase deficient VLCAD(-/-) mice. | |
| Biochim Biophys Acta. 2014; 1842: 677-85. | |
| Luedde M, Lutz M, Carter N, Sosna J, Jacoby C, Vucur M, Gautheron J, Roderburg C, Borg N, Reisinger F, Hippe HJ, Linkermann A, Wolf MJ, Rose-John S, Lüllmann-Rauch R, Adam D, Flögel U, Heikenwaelder M, Luedde T, Frey N. | |
| RIP3, a kinase promoting necroptotic cell death, mediates adverse remodeling after myocardial infarction. | |
| Cardiovasc Res. 2014; 103: 206-16. | |
| Bönner F, Borg N, Jacoby C, Temme S, Ding Z, Flögel U, Schrader J. | |
| Ecto-5'-nucleotidase on immune cells protects from adverse cardiac remodeling. | |
| Circ Res. 2013; 113: 301-12. | |
| | |
| Flögel U, Su S, Kreideweiß I, Ding Z, Galbarz L, Fu J, Jacoby C, Witzke O, Schrader J. | |
| Noninvasive detection of graft rejection by in vivo 19F MRI in the early stage. | |
| Am J Transplant. 2011; 11: 235-44. | |
| | |
| Mersmann J, Habeck K, Latsch K, Zimmermann R, Jacoby C, Fischer JW, Hartmann C, Schrader J, Kirschning CJ, Zacharowski K. | |
| Left ventricular dilation in toll-like receptor 2 deficient mice after myocardial ischemia/reperfusion through defective scar formation. | |
| Basic Res Cardiol. 2011; 106: 89-98. | |
| | |
| Pissarek M, Meyer-Kirchrath J, Hohlfeld T, Vollmar S, Oros-Peusquens AM, Flögel U, Jacoby C, Krügel U, Schramm N. | |
| Targeting murine heart and brain: visualisation conditions for multi-pinhole SPECT with 99mTc- and 123I-labelled probes. | |
| Eur J Nucl Med Mol Imaging. 2009; 36: 1495-509. | |
| Luedde M, Flögel U, Knorr M, Grundt C, Hippe HJ, Brors B, Frank D, Haselmann U, Antony C, Voelkers M, Schrader J, Most P, Lemmer B, Katus HA, Frey N. | |
| Decreased contractility due to energy deprivation in a transgenic rat model of hypertrophic cardiomyopathy. | |
| J Mol Med. 2009; 87: 411-22. | |
| Meyer-Kirchrath J, Martin M, Schooss C, Jacoby C, Flögel U, Marzoll A, Fischer J, Schrader J, Schrör K, Hohlfeld T | |
| Overexpression of prostaglandin EP3 receptors activates calcineurin and promotes hypertrophy in the murine heart. | |
| Cardiovasc Res. 2009; 81: 310-8. | |
| Levkau B, Schäfers M, Wohlschlaeger J, von Wnuck Lipinski K, Keul P, Hermann S, Kawaguchi N, Kirchhof P, Fabritz L, Stypmann J, Stegger L, Flögel U, Schrader J, Fischer J, Hsieh P, Ou YL, Mehrhof F, Tiemann K, Ghanem A, Matus M, Neumann J, Heusch G, Schmid KW, Conway EM, Baba HA. | |
| Survivin determines cardiac function by controlling total cardiomyocyte number. | |
| Circulation. 2008; 117: 1583-93. | |
| Westermann D, Mersmann J, Melchior A, Freudenberger T, Petrik C, Schaefer L, Lullmann-Rauch R, Lettau O, Jacoby C, Schrader J, Brand-Herrmann SM, Young MF, Schultheiss HP, Levkau B, Baba HA, Unger T, Zacharowski K, Tschope C, Fischer JW. | |
| Biglycan is required for adaptive remodeling after myocardial infarction. | |
| Circulation. 2008; 117: 1269-76. | |
| Flögel U, Laussmann T, Gödecke A, Abanador N, Schäfers M, Fingas CD, Metzger S, Levkau B, Jacoby C, Schrader J. | |
| Lack of myoglobin causes a switch in cardiac substrate selection. | |
| Circ Res. 2005; 96: e68-75. | |
| Stegger L, Schäfers KP, Flögel U, Livieratos L, Hermann S, Jacoby C, Keul P, Conway EM, Schober O, Schrader J, Levkau B, Schäfers M. | |
| Monitoring left ventricular dilation in mice with PET. | |
| J Nucl Med. 2005; 46: 1516-21. | |
| Martin M, Meyer-Kirchrath J, Kaber G, Jacoby C, Flögel U, Schrader J, Ruther U, Schrör K, Hohlfeld T. | |
| Cardiospecific overexpression of the prostaglandin EP3 receptor attenuates ischemia-induced myocardial injury. | |
| Circulation. 2005; 112: 400-6. | |
| Limbourg FP, Ringes-Lichtenberg S, Schaefer A, Jacoby C, Mehraein Y, Jager MD, Limbourg A, Fuchs M, Klein G, Ballmaier M, Schlitt HJ, Schrader J, Hilfiker-Kleiner D, Drexler H. | |
| Haematopoietic stem cells improve cardiac function after infarction without permanent cardiac engraftment. | |
| Eur J Heart Fail. 2005; 7: 722-9. | |
| Jacoby C, Molojavyi A, Schrader J, Flögel U, Meyer-Kirchrath J, Hohlfeld T. | |
| Cardiac MRI of transgenic mice. | |
| Bruker Spin Report. 2004; 154/155: 49-53. | |
| Warskulat U, Flögel U, Jacoby C, Hartwig HG, Thewissen M, Merx MW, Molojavyi A, Heller-Stilb B, Schrader J, Häussinger D. | |
| Taurine transporter knockout depletes muscle taurine levels and results in severe skeletal muscle impairment but leaves cardiac function uncompromised. | |
| FASEB J. 2004; 18: 577-9. | |
| Schlieper G, Kim JH, Molojavyi A, Jacoby C, Laussmann T, Flögel U, Gödecke A, Schrader J. | |
| Adaptation of the myoglobin knockout mouse to hypoxic stress. | |
| Am J Physiol Regul Integr Comp Physiol. 2004; 286: R786-92. | |
| Gödecke A, Molojavyi A, Heger J, Flögel U, Ding Z, Jacoby C, Schrader J. | |
| Myoglobin protects the heart from inducible nitric-oxide synthase iNOS-mediated nitrosative stress. | |
| J Biol Chem. 2003; 278: 21761-6. | |
|
|||