
 |
| | ||
| The molecule | Myoglobin | |
| 1H NMR | ||
| NO | ||
| Publications | ||
|
The molecule |
||||
|
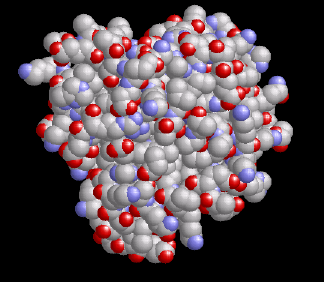
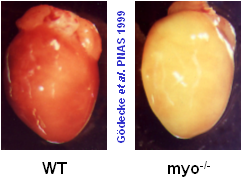
Myoglobin is a small, globular hemoprotein consisting of 153 amino acids (Mr ~17000 Da) found in the cytoplasm of skeletal (type I and IIa) and heart muscle in concentrations up to 0.5 mM. Myoglobin-containing muscles are red, such as a normal mouse heart (WT=wild type) shown at the right; if the myoglobin content is reduced, as in poultry meat, or if myoglobin is lacking completely due to a genetic manipulation (myo-/-, figure at the very right), the muscle fibers look much paler.
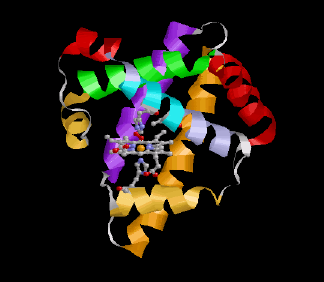
Similar to its molecular relative hemoglobin, which manages the O2 transport from the lung to the cells, myoglobin reversibly binds O2 but with a sixfold higher affinity than the – with the bloodstream circulating – hemoglobin. Thereby, it can easily take over the hemoglobin-provided O2 from the capillaries. O2 binds to the central iron atom of the heme group (marked in orange at the left; also compare the section about 1H NMR). For the oxymyoglobin so formed three different functions were up to now suggested, which were mainly derived from in vitro experiments with pharmacological inhibition of myoglobin:
|
|
||
 Functions of myoglobin
Functions of myoglobin
|
||||
|
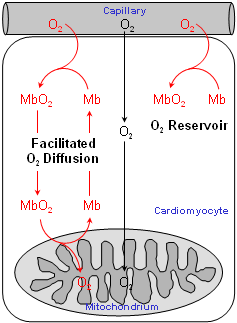
While the function of myoglobin as O2 reservoir is generally accepted – which is in particular supported by the up to tenfold higher myoglobin content in muscles of diving vertebrates, such as dolphins and whales – both of the other functions were under dispute for decades due to contradictory data in the literature. The entire research area was revived some years ago by the generation of myoglobin-deficient mice (see figure top right) in our and an american laboratory. By comparison of normal control animals with the myoglobin lacking mutant we could show, that the facilitated O2 diffusion by myoglobin indeed plays a major role for the adequate O2 supply of the heart, and that on the other hand the myoglobin-mediated oxidative phosphorylation is only, if at all, of less significance (Merx et al, 2001). What's more, we could discover surprisingly two important novel functions of myoglobin in muscle metabolism: The ability of myoglobin to effectively degrade both nitric oxide (NO) as well as free reactive oxygen species (compare also the original publications given below). The key to all of these investigations was the noninvasive measurement of myoglobin in the heart by 1H NMR spectroscopy (see next section). |
||||
| Top |
1H NMR spectroscopy of valine 68 |
|
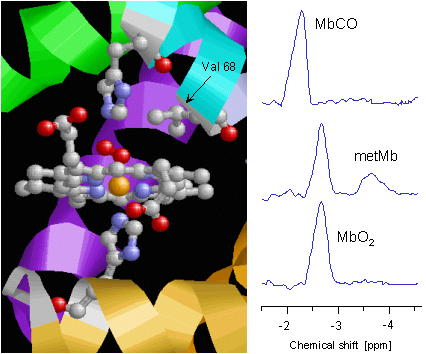
The adjoining figure shows once again in detail the structure of myoglobin's heme group. The central iron atom (orange) is surrounded by four nitrogen atoms (blue) in the plane of the porphyrin ring. The lower coordination site of the iron is occupied by another nitrogen (of the "proximal" histidine, His93 or F8), whereas the O2 bond takes place above the ring plan. The binding of O2 is additionally stablized by a hydrogen bridge bond to the "distal" histidine (His64 or E7). In the immediate neighbourhood one can recognize the methyl groups of valine 68 (E11), whose protons can be used as reporter nuclei of the current situation at the heme.
When the heart tissue is adequately supplied with O2, myoglobin – owing to its high O2 affinity – is completely oxygenated (MbO2), and the methyl protons of the valine can be detected at a chemical shift of -2.7 ppm in the 1H NMR spectrum of the heart (bottom trace). Only at a mismatch between O2 supply and consumption myoglobin gets deoxygenated which is reflected in an intensity decrease of the MbO2 signal. In that, the magnitude of this signal permits an assessment of the myocardial O2 supply. Making use of a simple mathematical transformation the intensity of MbO2 signal can be utilized to calculate the intracellular O2 partial pressure (PO2) of the heart muscle cells – a quantity, which is very difficult to access with other methods. |
|
|
The oxygenation level is, however, not the only parameter, which affects the signal of the valine protons in the 1H NMR spectrum. Generally spoken, each change of the chemical or electronical environment at the heme results in an alteration of the signal's intensity or position. For example, when the poisonous CO ejects the bound O2 due to its higher affinity to the heme iron, the formed MbCO is reflected in the spectrum by a signal at -2.3 ppm (top trace). Besides a ligand exchange the oxidation state of the iron also has an affect on the proton signal: if the iron in the center of myoglobin's heme looses an electron and converts from the normally divalent to the trivalent state – generally termed as metmyoglobin (metMb) – the fraction of the generated metMb can be unambiguously detected by its signal at -3.8 ppm (middle trace, see also the next section aboout myoglobin and nitric oxide).
Taken together, monitoring the protons of the valine 68 by 1H NMR spectroscopy as reporter nuclei of the electronic situation at the heme group yields versatile information about oxygenation, ligands, and redox state of cardiac myoglobin, and thereby about the current metabolic state of the heart. |
||
| Top |
Myoglobin and nitric oxide |
|
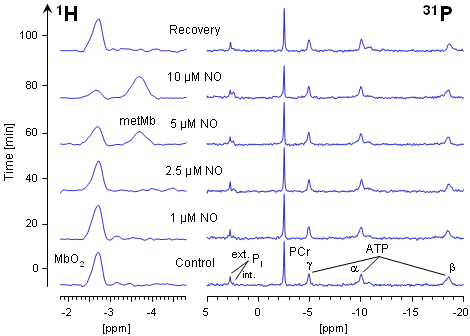
The figure on the left shows 1H and 31P NMR spectra of a wild type (WT) heart, to which continuously increasing amounts of nitric oxide were applied via the coronary vessels. As apparent from the both lower traces, NO concentrations up to 1 µM did neither affect the oxygenation of myoglobin nor the energy state of the heart. If the infused amount of NO was raised, at [NO] ≥ 2.5 µM a decrease of the MbO2 signal at -2.7 ppm was observed. Concomitantly, a new signal at -3.8 ppm was detected, that can be unequivocally assigned to metMb (see above) by both control measurements of commercially available Mb and comparison with literature data.
At [NO] = 5 µM approximately 50% of the MbO2 was converted to metMb reaching nearly 100% conversion at 25 µM NO (see also the quantitative analysis of this experiment). This reaction was reversible after cessation of the NO infusion (top trace), reflecting the rapid regeneration of MbO2 by metMb reductase activity (compare the scheme below). It is noteworthy that the conversion of MbO2 to metMb is already detectable at NO concentrations at which 31P NMR spectra did not yet show any adverse effect on cardiac energy status (third trace from top). |
|
| Further experiments about the dose-dependent effect of NO on function and energetics of WT and myoglobin-deficient (myo-/-) heart revealed, that exactly in the NO concentration range that caused the formation of metMb, myo-/- hearts were more sensitive to NO, in that depression of contractile as well as energetic parameters were more pronounced when compared with WT hearts (see also the quantitative analysis of this experiment). In an additional set of experiments we could show that lack of myoglobin results not only in an enhanced sensitivity of the heart to exogenously applied NO but also to the endogenous stimulation of the endothelial NO synthase (eNOS) by bradykinin (cf. Flögel et al, 2001). These data indicate that Mb is crucial to the inactivation of NO and substantially determines the doseresponse curve of the NO effects on coronary blood flow and cardiac contractility. | ||
|
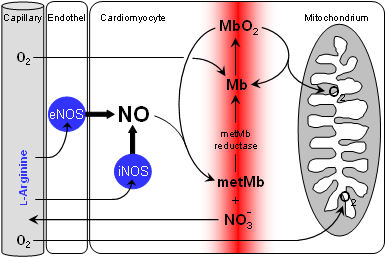
The described results can be easily reconciled with well-known chemical reactions of myoglobin and NO (see scheme on the right). However, although the individual steps have been exhaustively studied in vitro, it was not clear that together they constitute a metabolic cycle that has functional relevance in cardiac NO homeostasis in vivo. Obviously, the reaction of MbO2 + NO → metMb + nitrate represents a "chemical hoover", which takes care that even during enhanced NO synthase (NOS) activity cytosolic NO levels are kept extremely low, and by this protects the respiratory chain from detrimental effects of the reactive NO species.
Of fundamental importance for the efficacy of this hoover is the activity of the endogenous metMb reductase which ensures the regeneration of Mb from metMb. Subsequent association with O2 leads to reformation of MbO2 available for another NO degradation cycle. This "molecular firewall" works highly efficient, so that even a massively raised NO formation by an overexpression of the inducible NO synthase (iNOS) can suprisingly be neutralized. |
|
|
| The detrimental effects of durable increased amounts of NO surface not until myoglobin is inactivated in parallel by pharmacological or genetic intervention. Then they result –- similar as described above –- in impaired cardiac function and energetics, which lately may lead to terminal heart failure (cf. the original publications given below). Taken together these experiments demonstrate that myoglobin serves as an important cytoplasmic scavenger of bioactive NO, and thereby as a barrier for protection of the heart against nitrosative stress. This represents an additional –- besides its known role in oxygen storage and delivery –-, up to now hidden, physiological function of this well known protein. | ||
| Top |
Own work about the role of myoglobin in the heart |
| A complete overview about our peer-reviewed publications of the last years can be found here. The references are linked with the PubMed abstracts of the National Library of Medicine. If your are interested in one of these papers, and you don't have online access to the respective journal, send us an email, so that we can provide you with the appropriate pdf-file. |
| Methodical studies |
| Flögel U, Merx MW, Gödecke A, Decking UK, Schrader J. |
| Myoglobin: A scavenger of bioactive NO. |
| Proc Natl Acad Sci USA. 2001; 98: 735-40. |
| Merx MW, Flögel U, Stumpe T, Gödecke A, Decking UK, Schrader J. |
| Myoglobin facilitates oxygen diffusion. |
| FASEB J. 2001;15: 1077-9. |
| Reviews and Comments |
| Flögel U, Fago A, Rassaf T. |
| Keeping the heart in balance: the functional interactions of myoglobin with nitrogen oxides. |
| J Exp Biol. 2010; 213: 2726-33. |
| Hendgen-Cotta UB, Flögel U, Kelm M, Rassaf T. |
| Unmasking the Janus face of myoglobin in health and disease. |
| J Exp Biol. 2010; 213: 2734-40. |
| Flögel U, Dang CV. |
| Myoglobin tames tumor growth and spread. |
| J Clin Invest. 2009; 119: 766-8. |
| Applications |
| Hendgen-Cotta UB, Esfeld S, Coman C, Ahrends R, Klein-Hitpass L, Flögel U, Rassaf T, Totzeck M. |
| A novel physiological role for cardiac myoglobin in lipid metabolism. |
| Sci Rep. 2017; 7: 43219 (13 pages). |
| |
| Flögel U, Jacoby C, Gödecke A, Schrader J. |
| In vivo 2D mapping of impaired murine cardiac energetics in NO-induced heart failure. |
| Magn Reson Med. 2007; 57: 50-8. |
| Rassaf T, Flögel U, Drexhage C, Hendgen-Cotta U, Kelm M, Schrader J. |
| Nitrite reductase function of deoxymyoglobin: oxygen sensor and regulator of cardiac energetics and function. |
| Circ Res. 2007; 100: 1749-54. |
| Flögel U, Laussmann T, Gödecke A, Abanador N, Schäfers M, Fingas CD, Metzger S, Levkau B, Jacoby C, Schrader J. |
| Lack of myoglobin causes a switch in cardiac substrate selection. |
| Circ Res. 2005; 96: e68-75. |
| Merx MW, Gödecke A, Flögel U, Schrader J. |
| Oxygen supply and nitric oxide scavenging by myoglobin contribute to exercise endurance and cardiac function. |
| FASEB J. 2005; 19: 1015-7. |
| Flögel U, Gödecke A, Klotz LO, Schrader J. |
| Role of myoglobin in the antioxidant defense of the heart. |
| FASEB J. 2004; 18: 1156-8. |
| Wunderlich C, Flögel U, Gödecke A, Heger J, Schrader J. |
| Acute inhibition of myoglobin impairs contractility and energy state of iNOS-overexpressing hearts. |
| Circ Res. 2003; 92: 1352-8. |
| Gödecke A, Molojavyi A, Heger J, Flögel U, Ding Z, Jacoby C, Schrader J. |
| Myoglobin protects the heart from inducible nitric-oxide synthase iNOS-mediated nitrosative stress. |
| J Biol Chem. 2003; 278: 21761-6. |
| Gödecke A, Flögel U, Zanger K, Ding Z, Hirchenhain J, Decking UK, Schrader J. |
| Disruption of myoglobin in mice induces multiple compensatory mechanisms. |
| Proc Natl Acad Sci USA. 1999; 96: 10495-500. |
|
|||